Every two years, the Center for Information and Study on Clinical Research Participation (CISCRP) conducts the largest research study summarizing perceptions surrounding clinical trials. Trial participants and would-be study volunteers consistently cite the time, cost and inconvenience associated with attending study visits as the leading non-medical contributor impacting their enrollment in – and dropout of – trials.
Greenphire leverages CISCRP data as a market research foundation for our growing patient convenience solution set. Since 2008, the company has partnered with leading innovators across the clinical trial industry to remove the financial and logistical burden impacting patients, as well as research sites who administer. During this time, we have processed reimbursements and stipends for nearly five million trial participants across the globe. This experience also enables our in-house clinical trial experts to derive insights stemming from payment breadth.

Taxability of Clinical Trial Participation
Ethical concerns regarding payments were negated in 2018 when the FDA issued guidance documenting that “paying research subjects in exchange for their participation is a common and, in general, acceptable practice.”
While removing financial impediments from clinical trials can improve the patient experience and improve recruitment and retention, stipends have come under increased scrutiny. Currently, these payments are categorized as taxable income – like lottery winnings, and depending on the individual’s personal circumstances, can jeopardize governmental programs such as food stamps. You can read more information regarding the taxation of clinical trial payments in this article by Greenphire’s Senior Tax Director, Arpeeneh Adamian.
Advocating for Change: New Legislation Could Make an Impact
Greenphire supports all legislative efforts to improve patient access to clinical trials.
Recently, Congress introduced the Clinical Trial Modernization Act (H.R. 8412) to tackle the financial challenges faced by individuals with cancer and other serious illnesses who take part in clinical trials. This bipartisan legislation includes a key provision that exempts from taxation the first $2,000 in payments from clinical trial sponsors and prevents these payments from affecting eligibility for programs like Medicaid. Additionally, the bill establishes legal protection for sponsors who provide such financial support, shielding them from both civil and criminal liability.
Greenphire Patient Payment Data: A Powerful Proxy to Quantify Tax Impact
Why the $2,000 threshold?
If you look at aggregated data from Greenphire clinical trial payments across 871 trials in the 2023 calendar year, just 2.4% of trials paid their trial participants in excess of $2,000. Thus, if you were to raise the tax threshold to $2,000, it would include the majority of study participants in clinical trials.
For nearly 20 percent of clinical trial participants across various studies who received payments exceeding $600—12 percent of whom were oncology patients—most, if not all, paid taxes on these payments in 2023 for their participation in clinical trials.
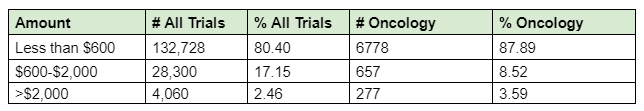
Methodology Statement: Categorized for clinical trials with payments issued within the 2023 calendar year, industry sponsored trials where sponsor and/or contract research organization preemptively requested Greenphire to support participant payment disbursements via Greenphire’s ClinCard solution. Payment specific data: inclusive of flat rate payment data excluding any travel-related expense reimbursement. Data across 871+ protocols with U.S. participation; amongst therapy areas and study phases I-IV. Inclusive of protocols that covered medical devices, pharmaceuticals, and biotechnologies.
What Next?
Approximately five percent of adults in the U.S. take part in clinical trials, but if we were to remove the taxability on payments for clinical trials, could that increase? Removing this burden would further increase patient access and potentially diversity, decrease burden on research sites for processing, and further streamline global study execution. A win-win-win for all study stakeholders, especially the most important one: Patients and their families.