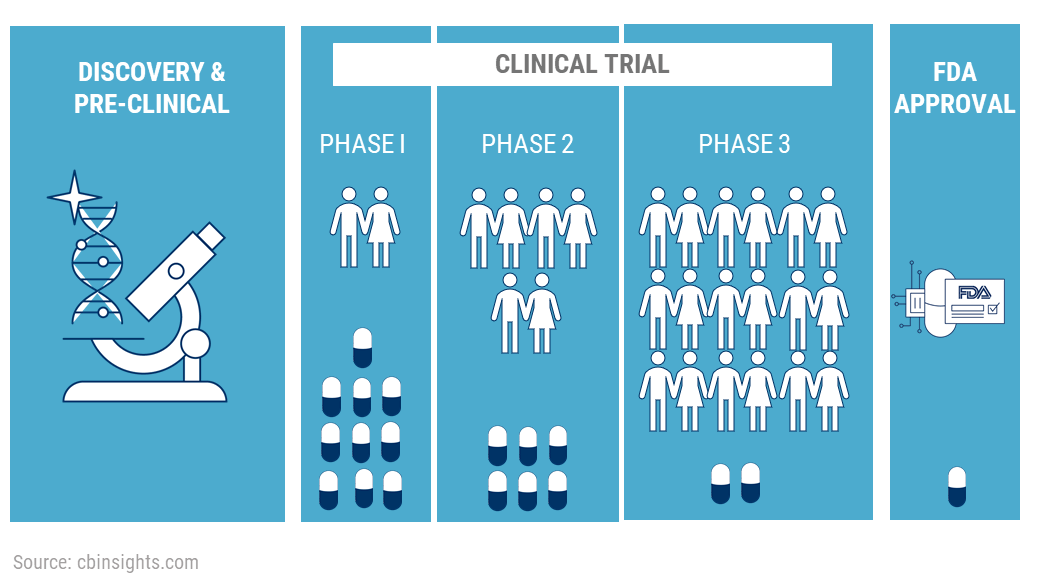
Clinical trials (CTs), while essential, are challenging to conduct and complete, and several unmet needs of all CT stakeholders are yet to be addressed. These needs and challenges range from better recruitment of trial investigators and patient enrolment to optimization of trial protocols and increased awareness about the importance of trials. Let’s look at some of these with regard to pharmaceutical/sponsors and participants.
CT-related challenges for pharma/sponsors
- Cost of developing a new drug
The expected mean estimate of bringing a new drug to the market is approximately $1.3 billion. However, the expenditure can range anywhere between less than $1 billion and more than $2 billion, including pre-clinical expenditures, capital costs, and the associated costs of new drugs that do not make it through the rigorous drug evaluation process.
- Sky-high costs of conducting CTs
The costs of pharmaceutical CTs largely depend on the therapeutic area which is being studied. A study averages the expenses of Phase 1 trials at a site in the US in the range of $1.4 to $6.6 million, $7.0 to $19.6 million for Phase 2 studies, and anywhere between $11.5 and $52.9 million for Phase 3 trials.
- Trial failures and delays
Most trials fail due to efficacy-related issues. Research studies reported that 54% of trials involving novel therapeutics fail in clinical development and 57% fail due to inadequate efficacy. Moreover, only 6% of trials are completed on time and more than 80% experience delays due to patient recruitment and retention, and compliance-related issues.
- Complex regulatory requirements
Numerous administrative and regulatory requirements at all stages of a CT can cause significant delays in its initiation. Moreover, regulations can vary across countries and regions (e.g., the latest EU regulations on CT summaries). Thus, when sponsor companies want to conduct multi-center/multi-national CTs, they must comply accordingly, put aside a separate budget for additional requirements, delegate the task to the most appropriate team, i.e., in-house or outsourcing via a CRO – clinical research organization.
- Time lags between protocol approval and trial activation
New CTs often take over six months from protocol approval to activation of the actual trial. Such protracted timelines incur heavy costs and decrease the overall trial efficiency.
- Difficulty enrolling patients
As stated above, many trials are terminated because they fail to accrue enough participants. Data even indicate that CT timelines have potentially doubled because of low recruitment rates. Finding, and screening suitable participants remains an ongoing challenge for the industry to address.
- Lack of training and administrative support
With the ever-increasing complexity of clinical research, lack of training and support for the clinicians and their staff may prove to be a deterrent. Furthermore, insufficient administrative assistance —for paperwork, clerical activities, data management, etc.—takes time away from their clinical practice, resulting in loss of earnings. Involvement in a CT would also require gatekeeping on behalf of the trial participants, which can be an added burden on the already busy site team. A form of intuitive payment automation for sites (supported by sponsors) would be a welcome step forward in this space.
CT-related challenges for patients
- Enrolment-related hesitancy
As stated above, a majority of CTs fail to recruit patients. The patient enrolment rate is usually affected by the following factors: Lack of awareness about the possibility of participating in a CT, narrow eligibility criteria and misunderstood expectations, fear and anxiety due to the uncertain nature of clinical research, distrust regarding industry-sponsored trials, difficulty locating a trial, confusion due to extensive paperwork associated with the informed consent process. Finally, the financial burden (for participants) can be difficult to navigate, alongside the rising cost of all forms of transportation..
- Continuation-related challenges
The patient dropout rate in CTs has historically been around 30%. Some common factors that contribute to this number are more frequent testing and the number of clinic visits required, inconvenient location of trial (traveling or commuting regularly can be a hurdle), patients not being able to choose which treatment they get in randomized double-blinded studies, treatment failure or underperformance, adverse side effects, and lack of appreciation.
In conclusion, there is room for improvement in the way CTs are conducted. Some ways to mitigate CT-related issues for all stakeholders are spreading awareness about CTs, integration of new technologies to improve trial processes participant reimbursement and travel payments, streamlining regulatory processes, adapting to emerging trial complexities, implementing better decision-making, as well as reducing costs and adopting innovative and efficient clinical practices to provide effective treatment to participants who are the backbone of CTs.