COVID-19 has had a dramatic impact on US clinical care, including how sites have worked to maintain research operations throughout the pandemic. According to the American Hospital Association, the financial impact of COVID-19 on the US healthcare system is estimated to total $325 billion in lost revenue in 2020 and the related negative impact has put downstream pressure on clinical research activities.
Our team at Greenphire recently conducted a webinar, The Impact of COVID-19 on Research Sites: Planning for the Road Back to Full Trial Operations.
The financial and logistical challenges faced by our site partners have been substantial, but I’m pleased to recap in this blog the adaptations they’ve made to manage through the current environment.
Adaptation #1: Increase of Remote Video Technology
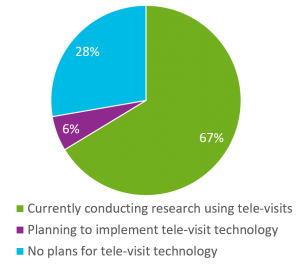
A significant adaptive technology across many sites was new implementation, or increased use, of telemedicine to maintain continuity of clinical care and to conduct clinical research visits remotely (due to site closures and the inability of research participants to travel). 67% of webinar attendees polled cited they are conducting research using tele-visits and another 6% were planning to implement the technology. Only 28% currently had no plans for tele-visits.
Adaptation #2: Remote Work
A second theme heard from sites was the realization that study coordinators and staff could conduct much of their study management activities remotely. Sites indicated a “hybrid work model” may be the “new norm” for some clinical research professionals, especially when coupled with remote video technology and remote informed consent and data collection tools.
Adaptation #3: Leveraging Multiple Digital Solutions
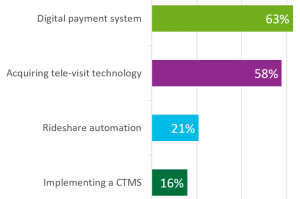
Given the increasing adoption of “remote study visit models”, a second audience poll question asked: which of four technologies (telehealth; digital participants payments; rideshare automation; or purchasing a CTMS) was a priority for sites based on the challenges presented by COVID-19. 63% indicated a digital payment system was their greatest priority; 58% indicated acquiring tele-visit technology; and less than 21% indicated rideshare automation or implementing a CTMS was a priority.
Adaptation #4: Rebounding from the Clinical Research Slowdown
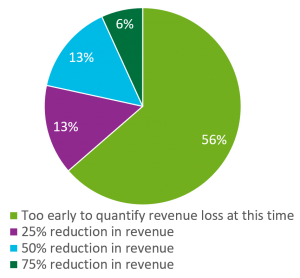
Although trials in the US were most significantly disrupted (paused or discontinued) in March and April, May thru July showed a trend of fewer trials being disrupted. An audience response question sought to measure the impact of these trial disruptions by asking sites to quantify the anticipated reduction in total clinical research revenue for 2020 as a result of the interruptions?
- 56% stated it was “too early to quantify revenue loss at this stage of the pandemic”
- 13% estimated a 25% reduction
- another 13% estimated a 50% reduction in revenue while 6% estimated a 75% reduction overall.
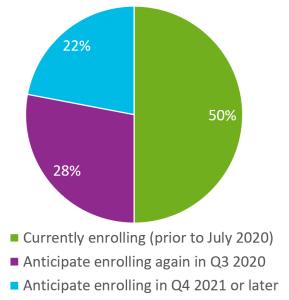
The panel also assessed the plans and timelines for sites to begin enrolling again and resuming trial operations. Here’s what sites said:
- 50% of sites said they were currently enrolling (prior to July)
- 28% anticipated enrolling again in Q3
- 22% planned to begin enrolling in Q4 or in 2021.
Adaptation #5: Participant Convenience and “Outside the Box” New Processes
COVID-19 has forced us the industry to truly put the patient first and embrace patient centricity overall. Technology and revamped trial processes will be the key to success in the second half of 2020 and beyond. Zach Hales, ClinCard Product Manager, shared how sites are increasingly leveraging tele-visit technology, electronic data capture, and being able to pay participants flexibly and digitally. Equally important and timely as a result of COVID-19 is patient travel; it’s essential that we are sensitive to the risk of virus transmission and offer other safe and clean services such as Greenphire-enabled rideshare versus crowed, unclean public transportation. Alternatively, the participant may be offered solutions to go to closer, nearby labs for tests or having home study visits made by research staff, where feasible.
Summary Observations:
The webinar concluded with a Panel discussion describing specific challenges Greenphire staff has been hearing from sites over the last few months. Andrew Glase, Account Executive, Clinical Research Sites, commented that non-client sites who were using gift cards or cash for participants’ payments were calling Greenphire to explain they were stuck and had to mail gift cards or cash. Many sites also recognized that tele-technology was the other major missing technology for sites, in addition to digital payments.
Lacey Kuberiet, ClinCard Product Trainer and Relationship Coordinator, explained that for university and academic medical center sites, “No one saw the pandemic coming. They had no way to prepare. But at least those who had ClinCard could continue making remote payments. Sites also made special arrangements for “pick-ups” to access the ClinCard for new enrollees and for life-sustaining studies”.
At Greenphire, and among our research partners, the COVID-19 pandemic has accentuated the need to communicate, introduce and share best practices to help sites overcome a very challenging time. Further adoption of new technologies that support digital trial management will become increasingly commonplace in the progression of industry best practices. Glase summarized the current situation in an accurate and generalizable way: “the current endeavor to get studies up and running at full scale is a shared priority among all sites, and new therapeutic solutions (like the urgency for a COVID vaccine or effective therapies) ultimately depend on perseverance and innovations from within the scientific research community. We have to keep going.”
The COVID-19 pandemic dictum ”we’re ALL in this together” clearly underlies what we believe at Greenphire in our commitment to support research site efficiency and continuous advancement of scientific discovery through clinical research.
If you were unable to attend the live webinar, we encourage you to watch it now.