Optimise Payment Workflows and Standardise Processes Across All Clinical Trial Stakeholders
Financial and administrative challenges in EU clinical trials are influenced by many factors, including region-specific business models and regulatory differences.
Automating key operational processes and introducing patient convenience solutions can introduce simplicity, bring transparency and retain clinical trial participants.
EU Clinical Trial Challenges
Clinical trials have become more complex and patient populations have become increasingly specialized. Coupled with delivering global services amongst a diverse set of stakeholders and manual processes, the study can suffer.
-
31%
OF TRIALS IN THE UK MET ENROLLMENT TARGETS
-
83%
OF SITES IN THE EU SAY THAT THEIR SITE’S INVOICING AND PAYMENT PROCESSES ARE PRIMARILY MANUAL
-
25%
OF SITES SPEND 7+ HOURS ON INVOICING ACTIVITIES PER MONTH
-
48%
OF EU SITES INDICATE THAT THEY ARE GETTING PAID LESS THAN 4 TIMES PER YEAR
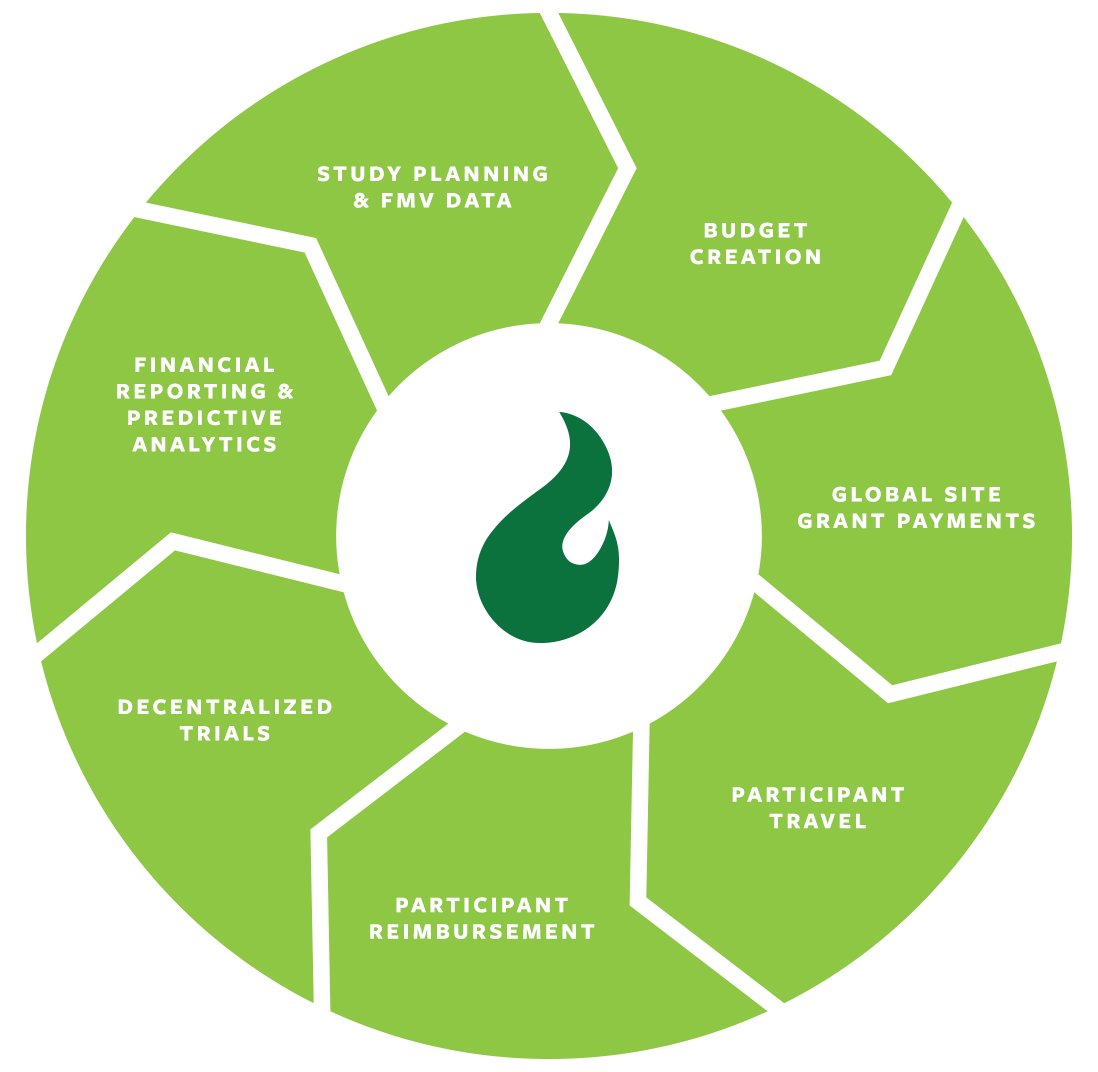
Clinical Trial Solutions to Optimize Your Studies
Technology solutions are enabling clinical trials to be conducted more efficiently and cost effectively, ultimately resulting in higher-quality data and better outcomes.
The realised benefits span all clinical trial stakeholders from sponsors and CROs to sites and participants.
Learn more about Greenphire’s solutions for patient convenience and financial transformation.

Regional Experience You Can Trust
Greenphire has deep experience executing patient reimbursement, travel and site payment solutions across the European continent.
- 1,800+ studies using Greenphire solutions
- 4,700+ European sites served
- 13,535 European participants reimbursed
- 30 European countries
Specialized EU Resources
What Sites Say
“eClinicalGPS saves a considerable amount of time in that I do not have to request the finance team to arrange the invoicing who then have to request the invoice from the credit team and this often results in delays.”
United Kingdom
Stand Out Thought Leadership
Where We’re Located
UK OFFICE
Sir Colin Campbell Building
University of Nottingham Innovation Park
Nottingham, NG7 2TU
United Kingdom
Contact Us Today for More Information or to Get a Demo!


Let Greenphire Help
Ready to see if Greenphire’s solutions can improve your clinical study site operations and patient convenience?