Transform Budget Development & Accelerate Study Startup
Financial and administrative complexities related to budgeting and contracting often delay clinical trials. Through Greenphire’s intuitive platform, sponsors, CROs, and research sites can simplify budget development and leverage our robust dataset to accelerate negotiations. Ready to drive increased efficiency and enhanced sponsor-site relationships by optimizing study startup processes? Let’s GO!
Get Ready to Run With Greenphire Clinical Trial Budgeting
Greenphire’s budgeting tool leverages the most comprehensive and current investigator grant actuals from clinical trials across the globe. Our data enables a superior budget negotiation starting point that sponsors and sites can trust, ultimately accelerating budget development and negotiation through amendments and finalization. See how it works in the video below.
Delivering Measurable ROI
Experience tangible benefits with Greenphire’s intuitive clinical trial budget development tool & contemporary source data:
-
2-week
reduction in budget creation timeline
-
20%
reduction in admin work
-
1-month
reduction in study startup timeline
What Our Clients are Saying
“Partnering with Greenphire allows us to unlock access to the most robust and contemporary investigator grant FMV data in the industry. This data, along with ease of building a budget through a simple and flexible tool, brings considerable value to our clients’ clinical development programs.”
Clareece West, Executive Vice President, Commercial Operations at Linical Americas.
Eliminate Guesswork From Your Clinical Trial Budgeting Process
Our solutions connect sites and sponsors, enabling you to achieve your joint goal of accelerating budget development and negotiations, with robust source data that both parties can trust and feel good about.
Benefit from our comprehensive platform features:
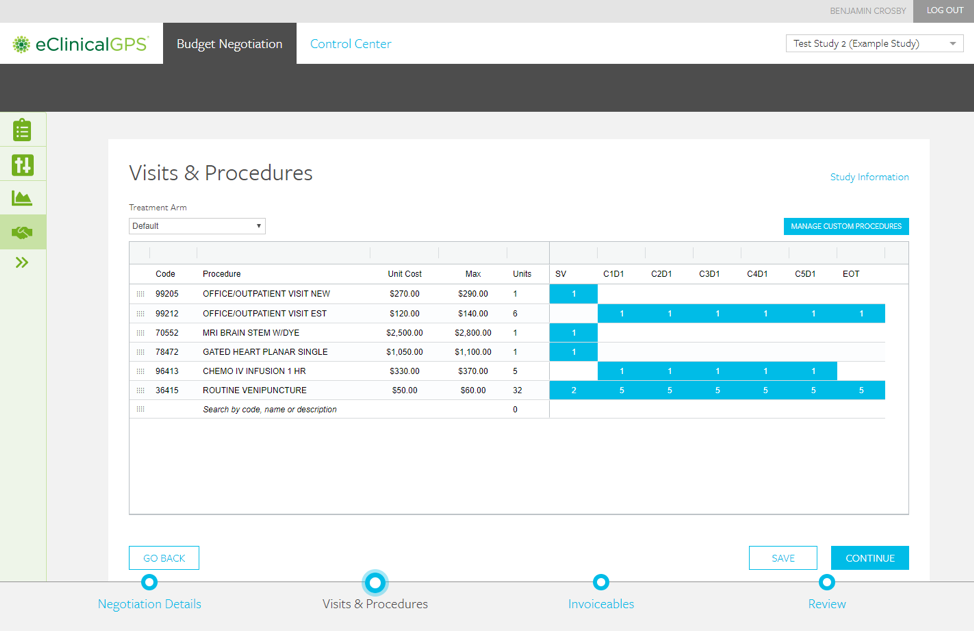
- Intuitive visit-schedule design
- Study, country, site levels
- Easy import of Protocol Schedule of Assessments
- Support of multiple treatment arms & cohorts
- Simple protocol & contract amendments
- Access to robust Site Payments FMV Data
- Exportable templates
- Integration with site payment solution
Enhance Your Experience Greenphire Clinical Trial Budgeting

Streamline Your Startup
Ready to accelerate budget development and negotiation timelines so you can get to study conduct sooner?
See our solutions in action.






